Off-the-Shelf Natural Killer (NK) Cells for Oncology
Natural Killer (NK) cells are essential components of the immune system, playing an important role in the body’s defensive response against tumors and infected cells. NK cells constantly patrol the body to detect and neutralize foreign cells. At Senti Bio, we believe there are several advantages that NK cells confer in relation to other potential immune cell types in oncology. These include innate killing and clinical activity, immune activation, previously validated efficacy and safety from over 900 patients, and broad patient access, as NK cells have the potential to be delivered rapidly to patients in an off-the-shelf manner and in outpatient settings.
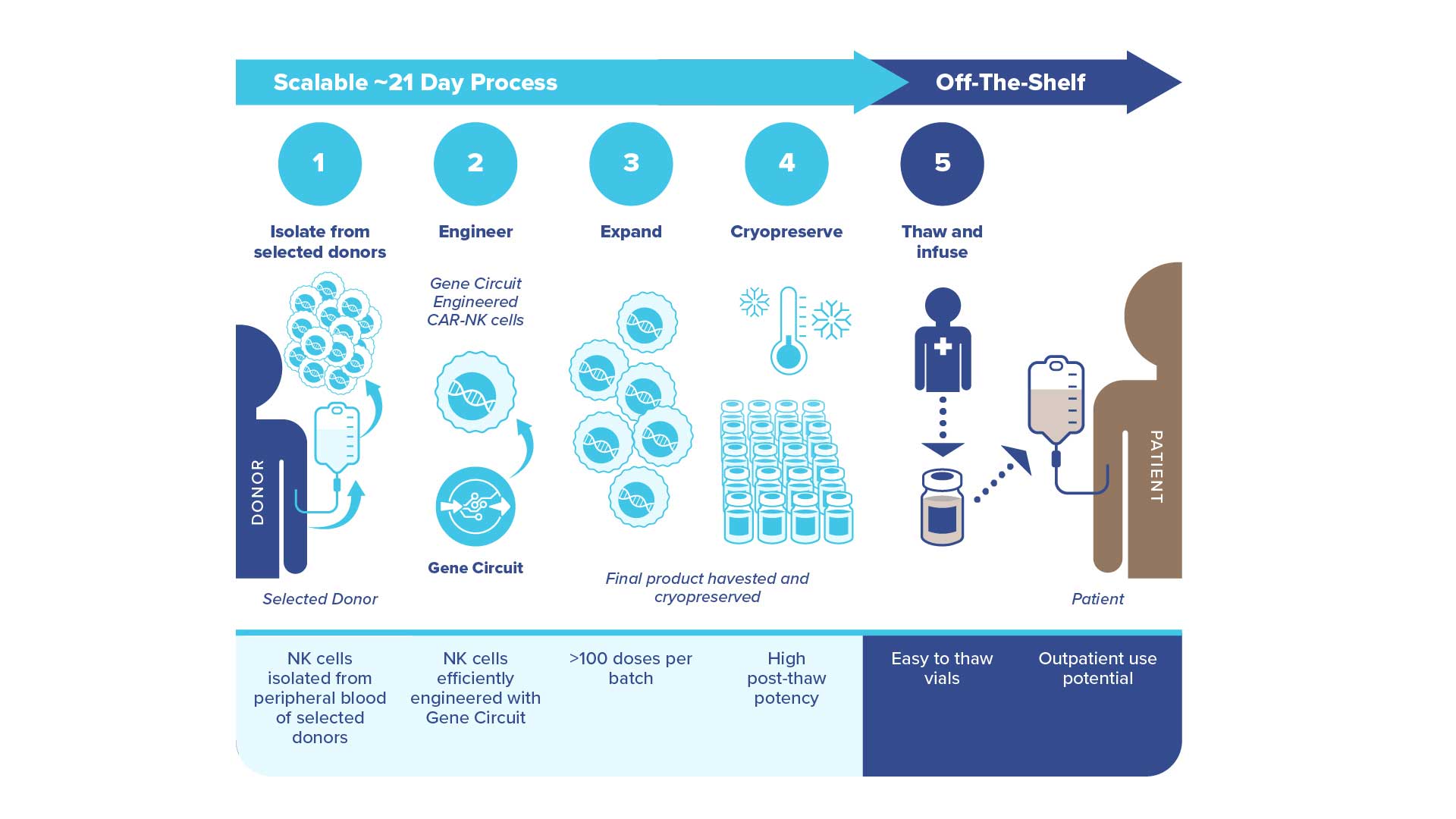
Our preferred cell source for our off-the-shelf CAR-NK cell pipeline is peripheral blood NK cells because this cell source allows us to immediately leverage an established supply chain, a mature GMP process and extensive clinical experience to develop our next generation CAR-NK cell therapies.
Strategic Manufacturing Capabilities Enable Production
of Off-The-Shelf Cell Therapies
Through our partnership with GeneFab, a contract manufacturing and synthetic biology biofoundry, we have the ability to control the quality and supply of our off-the-shelf CAR-NK cell therapy product candidates for clinical studies and ultimately commercialization. Senti screens healthy donors by enriching the cells from a healthy donor leukopak and evaluating the yield and growth potential of the NK cells. Donors who have NK cells that meet the criteria are selected for recall to provide a clinical grade leukopak for use in GMP manufacturing. A key potential advantage of off-the-shelf cell therapies, versus autologous products that use each patient’s own cells, is the ability to manufacture large batches of drug product from healthy donor cells that can be produced in advance of clinical use, and then stored in frozen vials. Upon commercialization, if our cell therapies are approved, we expect to be able to make these treatments broadly accessible in an off-the-shelf manner to cancer patients.